
ALCOA+ Principles in Pharma: The Next-Gen Standard for Data Integrity
ALCOA+ and the modern data integrity landscape
Data integrity underpins regulatory compliance and patient safety. And as pharmaceutical and biotech organizations adopt increasingly digital systems, automated workflows, and globalized supply chains, regulators including the FDA, EMA, and MHRA have intensified their focus on how data is generated, captured, and maintained.
The ALCOA+ principles form the recognized framework for ensuring that GxP data is trustworthy, traceable, and compliant throughout the lifecycle.
This guide outlines what ALCOA and ALCOA++ mean in practice, why they are central to inspection readiness, and how they can be embedded effectively across quality and regulatory systems.
- What ALCOA means for data integrity
- From ALCOA to ALCOA+
- The ALCOA+ principles and how to apply them
- Implementing ALCOA+ across systems
- ALCOA+ and regulatory compliance expectations
- Summary of ALCOA+ principles
- FAQs on ALCOA+ and data integrity
What ALCOA+ means for data integrity
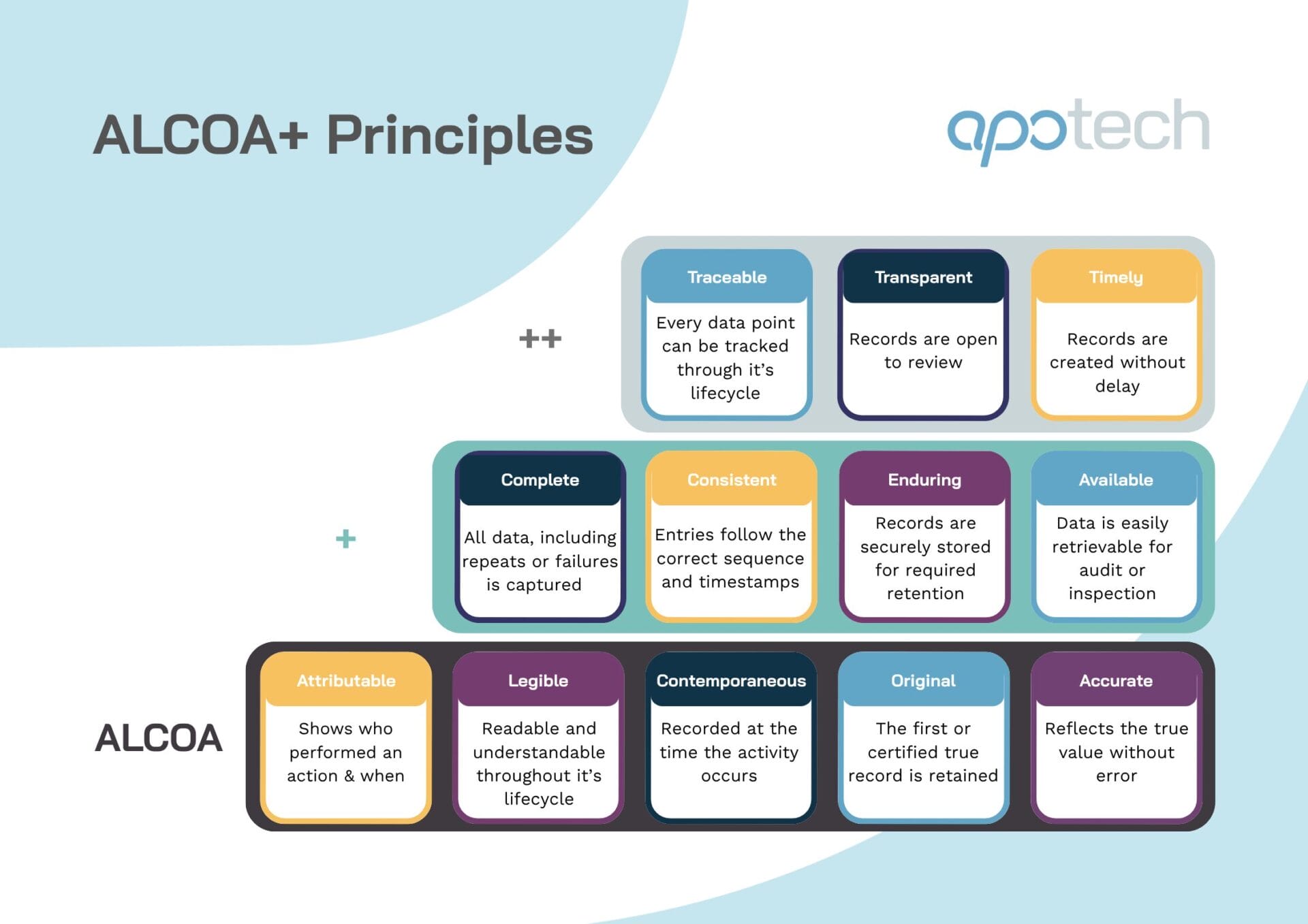
The ALCOA principles define the foundation of trustworthy data across regulated life sciences environments. First developed within Good Manufacturing Practice (GMP) to ensure that both paper-based and electronic records could be relied upon by regulators and stakeholders, ALCOA is now central to GxP compliance and inspection readiness.
The acronym ALCOA stands for:
- Attributable – Records must identify who performed an action and when.
- Legible – Data must be clear, permanent, and understandable throughout its retention period.
- Contemporaneous – Entries must be recorded at the time the activity is performed, not afterward.
- Original – The first capture of the data, or a certified true copy, must be retained.
- Accurate – Information must reflect the true observation or result, free from error or falsification.
These five elements are still the cornerstone of compliant documentation practices. However, as data management has evolved from manual to digital systems, new expectations have emerged for maintaining integrity throughout the entire data lifecycle.
From ALCOA to ALCOA+
As the life sciences industry adopted automated and electronic systems, regulators recognized that data integrity needed to extend beyond paper records to cover the entire data lifecycle. Traditional ALCOA principles ensured reliability in manual processes, but digitization introduced new challenges, from incomplete audit trails to inconsistent metadata, that the original framework could not fully address.
| Original ALCOA | ALCOA+/ALCOA++ |
| Paper-based records and manual data entry | Digital systems and automated data capture |
| Core principles: Attributable, Legible, Contemporaneous, Original, Accurate | Expanded attributes: Complete, Consistent, Enduring, Available, Traceable |
| Basic assurance of record reliability | Comprehensive, risk-based data governance model |
The ALCOA+ framework expanded on the original five principles to ensure that data is:
- Complete – capturing all results, including failed or repeat tests.
- Consistent – recorded in the correct sequence with aligned timestamps.
- Enduring – retained in durable, validated systems for the required period.
- Available – easily retrievable for review, audit, or inspection.
Some organizations have since adopted ALCOA++, adding further attributes that reflect the complexity of modern digital environments:
- Traceable – each data point can be followed through its lifecycle.
- Transparent / Timely (optional) – processes and records are open to review and created without unnecessary delay.
Together, ALCOA, ALCOA+, and ALCOA++ provide a lifecycle approach to data integrity, ensuring that information remains reliable, traceable, and inspection-ready across all GxP systems.
Regulatory authorities, including the FDA and EMA, now expect organizations to demonstrate:
- Controlled and reviewable audit trails
- Robust data governance
- Risk-based validation of computerized systems
These expectations are embedded in global guidance, underscoring the need for continuous oversight and proactive data governance in both GxP and non-GxP environments.
The ALCOA+ principles and how to apply them
Each principle supports a different aspect of reliable, inspection-ready data management. In practice, these principles guide how pharmaceutical and biotech organizations collect, store, and verify information to ensure regulators can trust every data point throughout its lifecycle.
The original ALCOA principles
Attributable
- Definition: Every data point must clearly identify who performed the task, including date, time, and system ID.
- Example: Electronic logbook entries record the user ID and timestamp for each action.
- Challenge: Shared user accounts or incomplete audit trails can obscure accountability.
- Checklist:
- Eliminate shared logins
- Apply role-based access controls
- Enable automated audit trail functionality
Legible
- Definition: Records must remain readable and accessible throughout their retention period.
- Example: Scanned batch records should be high-resolution and free from overwriting or corruption.
- Challenge: Poor-quality scans or obsolete file formats compromise long-term readability.
- Checklist:
- Use validated document management systems
- Preserve standardized, durable file formats
- Train users to maintain clarity in hybrid paper–digital records
Contemporaneous
- Definition: Record activities at the time they occur, not retrospectively.
- Example: Analysts enter results directly into validated systems during testing.
- Challenge: Backdating entries after a batch is complete creates compliance risk.
- Checklist:
- Use timestamped entries
- Synchronize system clocks
- Implement real-time data capture technologies
Original
- Definition: Retain the first capture of data, or a certified true copy, in a validated system.
- Example: Chromatogram files stored in their native electronic format.
- Challenge: Printing and discarding source files.
- Checklist:
- Store original data securely in validated repositories
- Maintain regular, verified backups
Accurate
- Definition: Data must reflect true results or observations, without manipulation or error.
- Example: Instrument calibration logs must align with actual usage dates and conditions.
- Challenge: Manual transcription errors or undocumented corrections.
- Checklist:
- Automate data capture wherever feasible
- Review and verify data entries regularly
The ALCOA+ principles
As data integrity expectations evolved beyond paper-based systems, regulators formalized ALCOA+, adding four new principles to ensure complete lifecycle control of electronic records.
Complete
- Definition: All data, including metadata and reprocessed or failed results, must be captured and retained.
- Example: All test runs, including failed or out-of-specification results, are stored.
- Challenge: Selective reporting or deletion of nonconforming results.
- Checklist:
- Preserve full audit trails
- Include metadata in retention policies
Consistent
- Definition: Data must follow a logical sequence with accurate timestamps and aligned time zones.
- Example: Batch records logged in chronological order across systems.
- Challenge: Unsynchronized system clocks or mismatched versions of records.
- Checklist:
- Apply system-wide clock synchronization
- Implement version control procedures
Enduring
- Definition: Records must be preserved in a durable, validated format for their required retention period.
- Example: Using validated archival storage that prevents data loss or format obsolescence.
- Challenge: Storing critical records on temporary or unvalidated drives.
- Checklist:
- Use validated data archives
- Periodically test the accessibility of archived data
Available
- Definition: Records must be readily retrievable for review, audit, or inspection throughout their lifecycle.
- Example: Inspectors can access all relevant records via a centralized document control system.
- Challenge: Fragmented storage systems that delay record retrieval.
- Checklist:
- Maintain centralized document control
- Define SOPs for retrieval and review
- Keep a complete data inventory
The ALCOA++ principles
In parallel, many organizations have also adopted ALCOA++, an industry-led extension that introduces further attributes focused on traceability and accountability in digital environments.
Traceable
- Definition: Every data point should be traceable from creation to archival, with a clear record of ownership and change history.
- Example: A validated LIMS records all user actions and modifications, including timestamps and reasons for change.
- Challenge: Weak version control or incomplete audit trails make it difficult to reconstruct data lineage.
- Checklist:
- Maintain linked audit trails for critical data
- Implement version control and change justification fields
- Include traceability checks in periodic integrity audits
Transparent / Timely (optional)
- Definition: Data processes should be open to review, and information should be generated promptly to reflect real-time activity.
- Example: Time-stamped entries are automatically visible in audit logs for QA review.
- Challenge: Manual data entry or delayed uploads obscure process visibility.
- Checklist:
- Enable automated timestamping
- Ensure audit trails are reviewable by QA
- Define SOPs for timely data entry
Implementing ALCOA+ across systems
To fully embed ALCOA+ principles, organizations should ensure that both paper-based and electronic systems demonstrate data integrity throughout their lifecycle. This means maintaining alignment between processes, technologies, and governance structures that underpin GxP operations.
Key focus areas:
- Audit trails and electronic signatures – Implement validated systems with complete, reviewable audit trails and compliant e-signatures.
- Time-synchronized clocks – Ensure all systems record timestamps consistently across networks and instruments.
- Metadata and raw data retention – Preserve complete data sets, including contextual information and associated metadata.
- Risk-based validation – Validate systems based on their impact on product quality and data integrity.
- Ongoing user training – Reinforce ALCOA+ principles through regular training, refresher programs, and inspection-readiness exercises.
5-step roadmap to operationalize ALCOA+
- Map your data flows – Identify where data is generated, transformed, and stored across all systems.
- Assess data integrity risk – Prioritize high-risk data sets and processes based on regulatory impact.
- Validate systems and audit trails – Confirm audit trail functionality, data retention, and user access controls.
- Embed ALCOA+ into SOPs and training – Incorporate principles into standard procedures and user onboarding.
- Monitor and trend findings – Conduct periodic audit trail reviews and track recurring issues to drive continuous improvement.
At Apotech, we support organizations in implementing practical, risk-based strategies for data integrity governance. Our consultants help map data flows, assess system validation readiness, and deliver training that embeds ALCOA+ principles across your operations, building confidence for both internal audits and regulatory inspections.
ALCOA+ and regulatory compliance expectations
Regulators such as the FDA, EMA, and MHRA continue to identify recurring data integrity issues across inspections, from incomplete audit trails to inadequate access controls. Embedding ALCOA+ principles within quality systems helps organizations prevent the compliance gaps that most often lead to Form 483 observations, warning letters, or critical inspection findings.
| Issue | Impact | Relevant ALCOA+ principle(s) |
| Deleted raw data | Compromises data integrity and traceability | Complete, Available |
| Shared user accounts | Obscures data ownership and accountability | Attributable |
| Inconsistent timestamps | Disrupts record chronology and verification | Consistent |
| Poor audit trial review | Allows undetected data manipulation | Enduring, Accurate |
Applying the ALCOA+ framework mitigates these risks by reinforcing traceability, accountability, and data completeness across all systems. It also demonstrates to regulators that data integrity controls are embedded into day-to-day operations, rather than applied retrospectively in response to findings.
Summary of ALCOA+ principles
The ALCOA+ framework builds on the original ALCOA principles to ensure data integrity across increasingly digital and interconnected life sciences systems. By embedding these principles, organizations can demonstrate that their data is complete, consistent, enduring, and available throughout the entire lifecycle, strengthening compliance, inspection readiness, and overall product quality.
- Lifecycle integrity: Extends beyond recordkeeping to ensure data remains reliable from creation to archival.
- Regulatory alignment: Recognized by the FDA, EMA, and MHRA as the benchmark for compliant data management.
- Digital readiness: Addresses the new risks introduced by automation, electronic systems, and global data environments.
- Data reliability: Reinforces that all records must be complete, consistent, enduring, and available throughout their lifecycle.
- Operational impact: Embedding ALCOA+ supports inspection readiness, strengthens governance, and builds regulator confidence.
Putting ALCOA+ into practice means having the right systems, validation, and governance in place. With our GxP audit services, we help you build data processes that stay compliant and inspection-ready as your organization grows. Get in touch today to discuss how we can support your next audit or inspection.
FAQs on ALCOA+ and data integrity
Some frameworks extend ALCOA+ to “ALCOA++” to include attributes like Traceable and Available. The “++” highlights continuous improvement in data governance.
Digital systems introduced new risks, including data migration, audit trail loss, and cloud retention, requiring broader principles for end-to-end integrity.
While not a standalone regulation, both the FDA and EMA reference ALCOA+ principles in their data integrity guidance, expecting them as best practice.
Begin with system validation, audit trail setup, and role-based access controls (RBAC). Regularly review data trends and conduct mock inspections to verify readiness.
Yes, ALCOA remains the foundation, but ALCOA+ provides the full framework for modern, compliant digital recordkeeping.