Good Laboratory Practice (GLP) & Good Clinical Laboratory Practice (GCLP) Audit Services
Our GLP audit and GCLP audit services support biotech and pharmaceutical organizations in assessing and strengthening compliance across laboratory, bioanalytical, and clinical laboratory environments. We work with sponsors, CROs, and laboratories to identify risk, protect data integrity, and demonstrate inspection readiness against global regulatory expectations.
What are GLP and GCLP audit services?
GLP and GCLP audit services evaluate whether laboratory-based activities supporting non-clinical and clinical development are conducted in compliance with applicable regulations, guidance, and sponsor expectations, including FDA and OECD GLP requirements and established GCLP principles. These audits assess quality systems, study conduct, data integrity controls, and operational practices that underpin the reliability of safety, biomarker, and other regulated data.
For organizations generating or relying on laboratory data, GLP / GCLP audits provide confidence that studies are planned, conducted, recorded, and reported in a manner that supports regulatory submissions and withstands inspection scrutiny.
Why GLP and GCLP audits matter
Failure to maintain robust GLP or GCLP compliance can result in:
- Regulatory findings related to data integrity, study conduct, or laboratory oversight
- Delays or rejections during IND, CTA, NDA, or MAA submissions
- Increased inspection risk during health authority audits
- Costly repeat studies or remediation activities
- Loss of confidence in critical non-clinical or clinical data

Our GLP / GCLP audit services offering
We provide a comprehensive, risk-based GLP / GCLP audit service across the full lifecycle of laboratory-based non-clinical and clinical studies.
Study and process audits
Audits focused on how studies are planned, conducted, controlled, and reported within GLP and GCLP environments.
This includes:
- Study audits (protocol adherence, conduct, deviations, reporting)
- Review of critical phases and processes (sample handling, analytical runs, data review, data release)
- Study report and raw data reviews (traceability, accuracy, scientific integrity)
Laboratory and facility audits
Assessment of the physical, organizational, and operational environment supporting regulated laboratory activities.
This includes:
- Facility inspections (infrastructure, equipment, utilities, security)
- Animal laboratory audits (welfare governance, husbandry, veterinary oversight)
Data integrity and systems audits
Targeted audits of data generation, handling, and control across paper-based and electronic laboratory systems.
This includes:
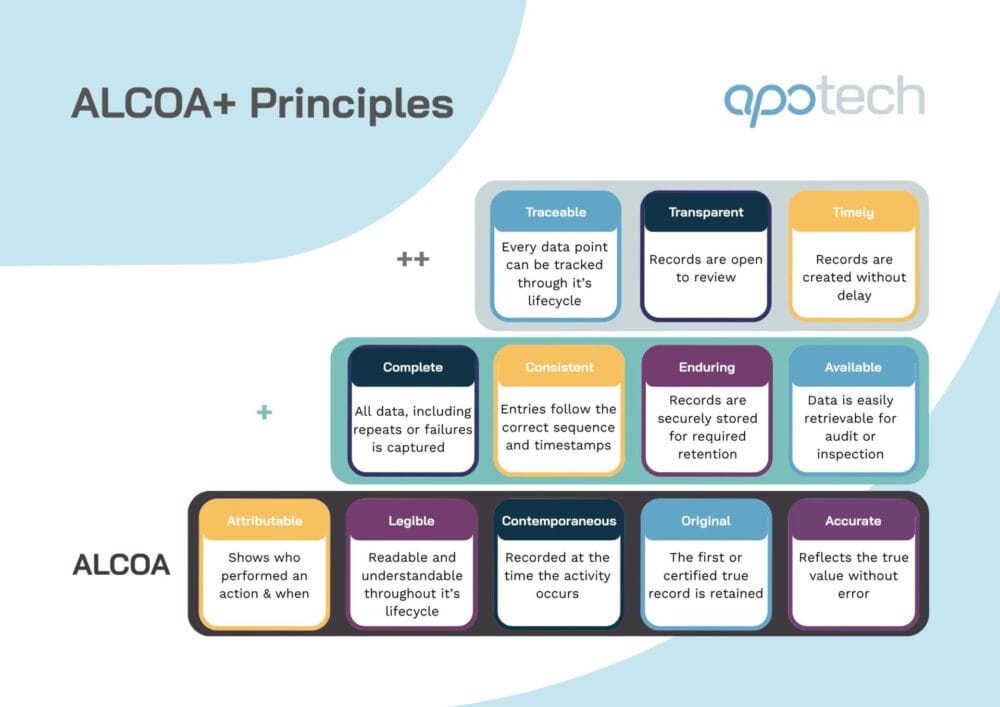
- Data integrity audits aligned to ALCOA+ principles
- Review of audit trails, system controls, and user access management
- Assessment of interfaces between analytical instruments, LIMS, and reporting systems
Documentation, archiving, and record lifecycle audits
Evaluation of documentation control and long-term record management supporting regulatory submissions and inspections.
This includes:
- Review of SOPs, training records, and quality documentation
- Archive audits covering record retention, retrieval, security, and disaster recovery
How our GLP / GCLP audit process works
Our GLP / GCLP audits are delivered using a structured, risk-based approach tailored to your development stage and regulatory exposure.
Step 1 - Scoping and risk assessment
We define audit scope based on study type, data criticality, regulatory submission plans, and prior inspection history.
Step 2 - Auditor assignment
An experienced GLP or GCLP auditor is assigned based on laboratory type, scientific discipline, and regional regulatory requirements.
Step 3 - Audit preparation
We prepare the audit plan, agenda, and document request list, engaging with the auditee to ensure a focused and efficient audit.
Step 4 - Audit execution
Audits are conducted onsite or remotely and include interviews, system reviews, and detailed assessment of study and laboratory practices.
Step 5 - Audit reporting
We deliver a clear, structured audit report presenting observations in context, categorized by risk and regulatory relevance, with a focus on study impact, data integrity, and inspection readiness.
Step 6 - Follow-up and ongoing support
Following report delivery, we remain available to support CAPA review, clarification with sponsors or vendors, and any required CAPA follow-up activity to ensure findings are understood, addressed, and effectively resolved.

Trusted by top-tier teams worldwide

















650+
Audits conducted in the last year
800
Network of expert GxP auditors
110
Countries covered
Why choose Apotech for GLP / GCLP audit services
We differentiate ourselves through technically rigorous, regulator-focused audit delivery supported by deep laboratory and study-level expertise.

Global GLP / GCLP specialists
Our global network of senior auditors includes specialists with hands-on experience across GLP and GCLP laboratory environments, including toxicology, bioanalysis, translational research, and clinical laboratories. This enables locally delivered audits aligned to both global regulatory expectations and regional practice.

Regulatory and laboratory expertise
Our auditors bring real-world experience from pharmaceutical, biotech, and CRO laboratory settings, supported by up-to-date knowledge of GLP regulations, GCLP principles, and guidance from authorities such as the FDA, EMA, and MHRA. They understand how regulators assess laboratory systems, study conduct, and data integrity in practice.

Cost-effective delivery
By using regionally based auditors, we minimize travel and associated costs while maintaining audit depth and technical rigor.

Flexible, client-centered support
We offer scalable GLP / GCLP audit solutions, from targeted study or facility audits to fully outsourced laboratory audit programs. Our dedicated Business and Project Manager model ensures clear communication, efficient scheduling, and appropriate alignment of auditor expertise.
Our Global Reach
We take pride in our extensive global audit network and are committed to utilising local auditors based in-country.
This approach offers several key benefits: local auditors possess in-depth knowledge of regional regulations, communicate effectively in the local language, and help reduce travel expenses for our clients. We have successfully provided support in all the regions highlighted on the map below.
Featured case studies
GxP Audits – Worldwide
GxP Audits – Worldwide Introduction Global Biopharmaceutical Our client is a global biotechnology company dedicated to novel treatments for patients through inhibition of protein kinases to fight cancer. They are Headquartered in the US, with a large footprint in the EU. Types of Audits GCP – For Cause / Investigator / Phase 1 unit GCLP – Central…
Regulatory Strategy IVDR / FDA
Regulatory Strategy IVDR / FDA Introduction Deciphex Deciphex is a digital pathology workflow and integrated AI platform for research pathology, CROs and pharmaceutical companies for handling high volume drug safety pathology studies. They provide a complete GLP-compliant workflow with integrated AI screening capabilities. Standards & Tools EU-IVDR 2017-746 ISO 13485 ISO 14971 IEC 62366 GDPR…
FAQs on Audits
We provide a modern approach to auditing that offers full transparency. We aim to make the process as simple to understand as possible and are always on hand to answer any questions you might have.
Here are some of the questions we get asked most often.
Compliance, standards & quality assurance
Our GLP / GCLP audit services are delivered in line with applicable regulatory requirements and guidance, including GLP regulations, GCLP principles, ICH guidance, and relevant regional expectations. All audits are conducted independently, objectively, and with strict confidentiality, with a clear focus on inspection readiness.
Data protection & confidentiality
We operate under robust data protection and confidentiality controls to safeguard sensitive study, patient, and proprietary information.
Contact us
Whether you need a full GLP / GCLP audit programme or targeted support for a specific laboratory or study, our team can help you strengthen compliance, protect data integrity, and prepare with confidence.
If you’re interested in our consultancy services, find out more about our QA services and get in touch today
Helpful resources
10 CAPA Metrics and KPIs Every Quality Team Should Track
By Imane Nohair, GxP Audit manager at Apotech Corrective and Preventive Actions (CAPAs) are a fundamental element of quality management across the pharmaceutical & biotechnology industries. When designed and executed effectively, CAPAs do more than address isolated non-conformities. CAPA processes strengthen quality systems, reduce regulatory risk, and support sustained compliance across global regulatory frameworks. Across…
ALCOA+ Principles in Pharma: The Next-Gen Standard for Data Integrity
ALCOA+ and the modern data integrity landscape Data integrity underpins regulatory compliance and patient safety. And as pharmaceutical and biotech organizations adopt increasingly digital systems, automated workflows, and globalized supply chains, regulators including the FDA, EMA, and MHRA have intensified their focus on how data is generated, captured, and maintained. The ALCOA+ principles form the…
GxP Compliance Explained: Beyond Best Practice
Understanding the meaning of GxP is essential for any organization operating in the life sciences sector. In simple terms, GxP refers to “Good Practice” quality and regulatory guidelines, used to ensure products are safe, effective, and reliable throughout their lifecycle. These standards apply across pharmaceuticals, biotechnology, medical devices, and laboratory environments, and are enforced by…