What are GMP and GDP audits?
GMP and GDP audits are structured, independent assessments of manufacturing and distribution systems that determine whether facilities, processes, and controls comply with applicable regulatory requirements. This includes alignment with EU GMP Parts I and II, EU GDP Guidelines, FDA expectations, and relevant ICH guidance.
GMP audits focus on how medicinal products, APIs, and investigational materials are manufactured, tested, and released. GDP audits assess how products are stored, handled, transported, and distributed through the supply chain. Together, they provide assurance that product quality, integrity, and traceability are maintained from manufacture through delivery, in line with MAH oversight obligations.
Why GMP & GDP audits matter
Failure to maintain robust GMP or GDP compliance can result in:
- Regulatory non-compliance can lead to inspection findings, enforcement actions, supply disruption, or license impact
- Weak supplier or distributor oversight increases the risk of product quality defects, temperature excursions, and recalls
- Inadequate records and Quality Management System (QMS) controls undermine batch release, investigations, and inspection readiness

Our GMP & GDP audit offering
We provide a comprehensive, end-to-end GMP and GDP audit service covering the following areas:
API and IMP manufacturing audits
Assessment of manufacturing and testing activities for APIs and investigational medicinal products, including:
- Process controls, validation, and change management
- Raw material qualification and supplier oversight
- Batch documentation, deviations, and investigations
Contract manufacturing organizations (CMOs / CDMOs)
Independent audits of outsourced manufacturing partners to support qualification and ongoing oversight:
- Quality agreements and responsibility mapping
- Technology transfer and lifecycle management
- Oversight models aligned with MAH obligations
Storage and distribution centers
GDP audits of warehouses, logistics providers, and distribution hubs, covering:
- Temperature control, monitoring, and excursion management
- Transportation qualification and cold chain integrity
- Traceability, returns, and recall readiness
Test laboratories
Assessment of GMP laboratories supporting manufacturing and release activities:
- Analytical method validation and data integrity
- Sample management and result reporting
- OOS, OOT, and investigation handling
Records and quality management systems
Cross-functional review of documentation and QMS maturity, including:
- SOP governance and training effectiveness
- Deviation, CAPA, and change control systems
- Data integrity and records management

How our GMP and GDP Audit process works
Step 1 - Scoping and needs assessment
We align on audit objectives, regulatory context, product types, and risk areas, ensuring the audit scope reflects your supply chain, inspection history, and regulatory priorities.
Step 2 - Auditor assignment
An experienced GMP/GDP auditor is assigned based on technical expertise, facility type, and relevant regulatory expectations.
Step 3 - Audit preparation
The auditor prepares the audit plan, agenda, and documentation request list, coordinating with the auditee to confirm scope and logistics.
Step 4 - Audit execution
The audit is conducted onsite or remotely and includes facility walkthroughs, interviews, and detailed review of systems, processes, and records.
Step 5 - Audit reporting
You receive an inspection-ready audit report with a clear compliance narrative and structured findings mapped to applicable regulatory requirements.
Step 6 - Follow-up and ongoing support
Following report delivery, we remain available to support CAPA review, and any required CAPA follow-up activity, re-audit support, and inspection readiness guidance to ensure findings are addressed and sustained over time.
Trusted by top-tier teams worldwide

















650+
Audits conducted in the last year
800
Network of expert GxP auditors
110
Countries covered
Why choose us for GMP and GDP audits?
At Apotech, our onsite and remote GMP and GDP audits support pharmaceutical and biotechnology organizations in maintaining compliant manufacturing and distribution operations and meeting evolving regulatory expectations. Our approach is pragmatic, risk-focused, and grounded in real-world GMP and GDP practice. We differentiate ourselves through:

Global GxP specialists
Our auditors have hands-on experience across global manufacturing and distribution environments, including clinical, commercial, and virtual supply chains.

Regulatory and technical expertise
We bring deep knowledge of GMP and GDP requirements, inspection trends, and regulator expectations across the FDA, EMA, and other global authorities.

Competitive, cost-effective delivery
Our risk-based approach focuses effort where it matters most, delivering high-quality audits without unnecessary complexity.

Flexible, client-centered service model
We adapt our audits to your operating model, maturity level, and internal processes, acting as a long-term partner rather than a transactional auditor.
Our Global Reach
We take pride in our extensive global audit network and are committed to utilising local auditors based in-country.
This approach offers several key benefits: local auditors possess in-depth knowledge of regional regulations, communicate effectively in the local language, and help reduce travel expenses for our clients. We have successfully provided support in all the regions highlighted on the map below.
Featured case studies
Outsourced internal GMP & GVP Audits
Outsourced internal GMP & GVP Audits Introduction Multinational biotech The French affiliate of a multinational Biotech company has its own QMS. Upon the QP responsibility, they conduct their internal audit with a 3-year plan (at the end of a 3 year cycle, they have audited all of their processes). Apotech consultants 2 External auditors Both…
GMP Audits
GMP Audits Introduction UK Based Biotech The company is a worldwide large biotech with a vast portfolio of biotech products (mainly oncology). Apotech auditors: 3 auditors are involved in this partnership. Types of Audits Contracts/agreements between the clients and the provider GMP/GDP Client SOPs Local regulations Audit Locations Europe The audited sites are: Client manufacturing…
FAQs on Audits
We provide a modern approach to auditing that offers full transparency. We aim to make the process as simple to understand as possible and are always on hand to answer any questions you might have.
Here are some of the questions we get asked most often.
A GMP audit is an independent assessment of manufacturing and quality systems to verify compliance with Good Manufacturing Practice requirements and applicable regulatory guidance.
Compliance, standards & quality assurance
Our GLP / GCLP audit services are delivered in line with applicable regulatory requirements and guidance, including GLP regulations, GCLP principles, ICH guidance, and relevant regional expectations. All audits are conducted independently, objectively, and with strict confidentiality, with a clear focus on inspection readiness.
Data protection & confidentiality
We operate under robust data protection and confidentiality controls to safeguard sensitive study, patient, and proprietary information.
Contact us
Whether you require a full GMP and GDP audit program or targeted support for a specific supplier or facility, our team can support your compliance objectives and inspection readiness.
If you’re interested in our consultancy services, find out more about our QA services and get in touch today
Helpful resources
How to Make Sure Your CAPA Process Actually Works?
By Imane Nohair, GxP Audit manager at Apotech If you work in quality management, you know how important the CAPA (Corrective and Preventive Action) process is. It helps identifying problems, fixing them, and preventing them from happening again. But here’s the real challenge: how do you make sure those fixes actually work? Many companies put…
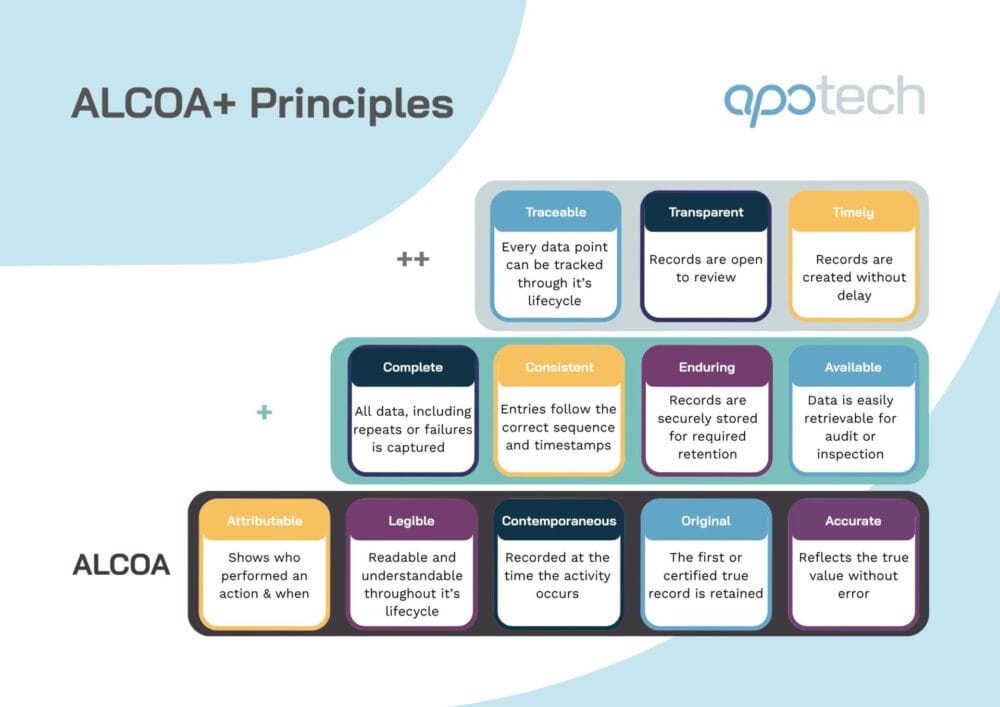
ALCOA+ Principles in Pharma: The Next-Gen Standard for Data Integrity
ALCOA+ and the modern data integrity landscape Data integrity underpins regulatory compliance and patient safety. And as pharmaceutical and biotech organizations adopt increasingly digital systems, automated workflows, and globalized supply chains, regulators including the FDA, EMA, and MHRA have intensified their focus on how data is generated, captured, and maintained. The ALCOA+ principles form the…
GxP Compliance Explained: Beyond Best Practice
Understanding the meaning of GxP is essential for any organization operating in the life sciences sector. In simple terms, GxP refers to “Good Practice” quality and regulatory guidelines, used to ensure products are safe, effective, and reliable throughout their lifecycle. These standards apply across pharmaceuticals, biotechnology, medical devices, and laboratory environments, and are enforced by…