Articles
At Apotech, we are fully aware that the patient is the ultimate stakeholder of our projects, so we are dedicated to meeting all of our customer’s requirements.
Featured Article
GxP Compliance Explained: Beyond Best Practice
Understanding the meaning of GxP is essential for any organization operating in the life sciences sector. In…
Finding the Right Quality Assurance (QA) Consultants in Life Sciences
Written by Connie Prince – Talent Acquisition Manager Life sciences organizations increasingly rely on Quality Assurance (QA)…
How To Master The CAPA Process Step-by-Step: From Identification to Implementation
The Corrective and Preventive Action (CAPA) process is a core mechanism within Pharmaceutical and Biotech quality systems,…
What is QMSR vs QSR? Understanding The FDA Final Rule and ISO 13485 Harmonization
The Quality Management System Regulation (QMSR) is the U.S. Food and Drug Administration’s (FDA) new rule replacing…
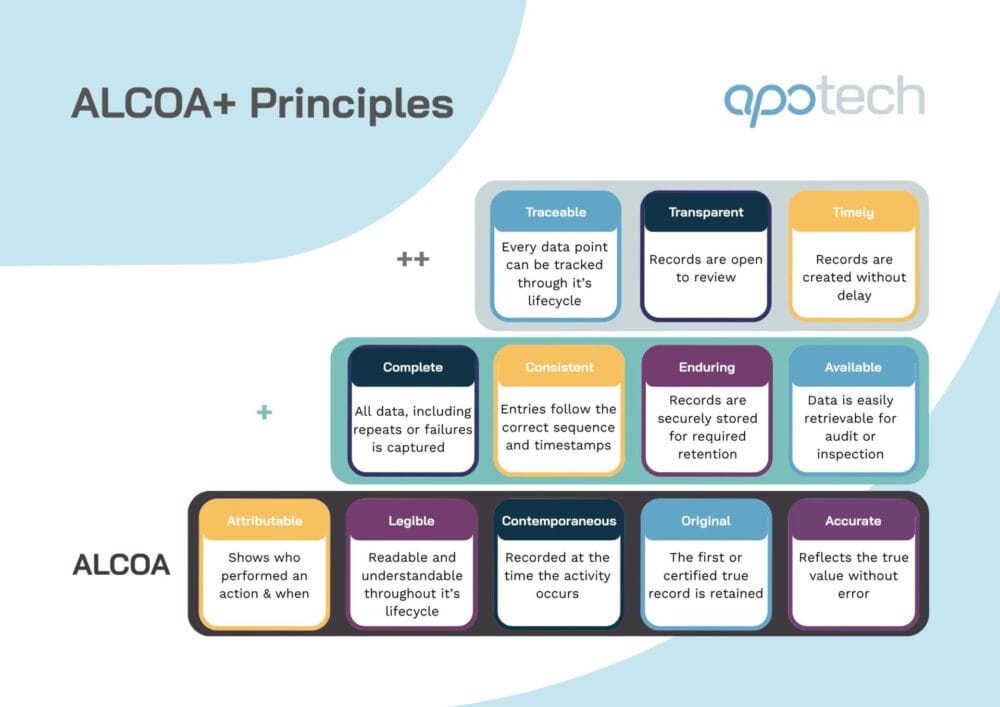
ALCOA+ Principles in Pharma: The Next-Gen Standard for Data Integrity
ALCOA+ and the modern data integrity landscape Data integrity underpins regulatory compliance and patient safety. And as…
Celebrating Global Partnerships: Apotech Consulting Expands to South Korea Partnering With Consulting BARO Inc.
At Apotech Consulting, international coverage has been part of our DNA since day one. Our mission has…
2024 Pharmaceutical Inspections: Risk-Based, Remote-Ready, and Globally Harmonized
Staying audit-ready is no longer optional, it’s strategic! Based on EFPIA’s 2024 Inspection Survey, pharmaceutical manufacturers must…
How to Make Sure Your CAPA Process Actually Works?
By Imane Nohair, GxP Audit manager at Apotech If you work in quality management, you know how…
From Audit Non-Conformities to CAPA: A Systematic Approach
By Imane Nohair, GxP Audit manager at Apotech An organized method from audit non-conformities to CAPA overview…
10 CAPA Metrics and KPIs Every Quality Team Should Track
By Imane Nohair, GxP Audit manager at Apotech Corrective and Preventive Actions (CAPAs) are a fundamental element…
Get ready for Pharmaceutical Audits 2.0: How Technology Is Having An Impac
By Imane Nohair, GxP Audit manager at Apotech Introduction In the highly regulated pharmaceutical industry, audit readiness…