
Truly ‘tailor-made’ flexible and bespoke solutions
Global Complexity, Simplified Locally
As global industry experts, Apotech helps life sciences organisations around the world streamline processes, mitigate risks and adhere to complex industry regulations.
We offer a modern and dynamic approach that delivers complete regulatory peace of mind and confidence in your compliance.
Pushing the boundaries of life sciences consulting
After assessing current complexities within international markets and regulatory processes, as well as the gradual integration of intelligent technologies, Apotech was created to meet the unique, ever-changing needs of the life sciences industry.
From Pharma companies to MedTech manufacturers to in vitro diagnostic (IVD) suppliers, our services have been designed to support organisations throughout the life sciences landscape, allowing cutting-edge medical solutions to reach those who need them most.
Pharma and Biotech
Apotech offers tailored comprehensive services catering to the Pharmaceutical and Biotech sectors, covering a broad spectrum from generics to cutting-edge biologics such as cell and gene therapies. Our core proficiency lies in deciphering global regulatory standards, whether originating from the US, EU, UK, or other regions worldwide, and seamlessly translating them into compliant solutions for you.
From intricate BLA submissions to specialised onsite GxP audits, and the establishment of biologic manufacturing validation master process, Apotech delivers customised solutions to meet your exact requirements. Our service portfolio includes:
MedTech
Our MedTech consulting services cover all stages, from pre-market preparation to manufacturing and post-market support. Our expertise enables you to achieve regulatory compliance seamlessly throughout your product lifecycle.
With frequent interaction with national competent bodies, and many of our consultants with experience within these organisations, Apotech brings first-hand insights and a personalised approach to support you in all international MedTech requirements to achieve regulatory compliance throughout your product lifecycle. We can provide support in the following areas:
In Vitro Diagnostics
At Apotech, we understand the unique challenges of the IVD industry. Our consulting services are tailored to support you at every stage of your journey, from pre-market planning to post-market surveillance.
With a deep understanding of IVD regulations and frequent engagement with national competent bodies, our team brings unparalleled expertise to help you navigate complex regulatory landscapes and achieve compliance throughout your IVD lifecycle. We can provide support in the following areas:
Audits in Pharma and Biotech
At Apotech, we empower life science companies and manufacturers to meet their regulatory duties and achieve operational excellence via a range of comprehensive auditing services.
Whether it be ensuring the integrity and quality conduct of your clinical trials, or bolstering the credibility of your data, our team streamline the auditing process to ensure complete regulatory compliance. We cover:
Representative Services
Enhance regulatory compliance of your medical devices or in vitro diagnostic (IVD) products by selecting Apotech as your UK Responsible Person, European Union Authorised Representative or Person Responsible for Regulatory Compliance.
Trusted by top-tier teams worldwide












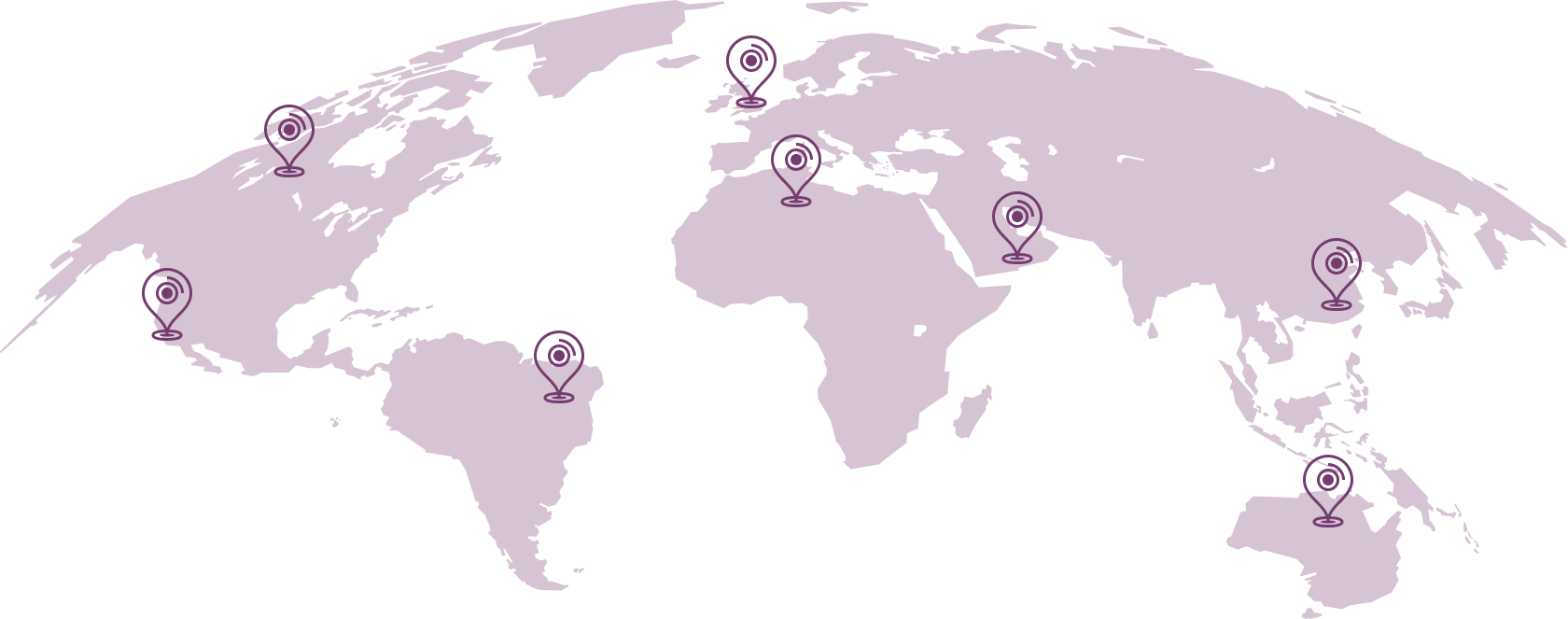
Our global presence spans across the world!
With consultants and teams present in 30 key markets across the world, our global presence is something we’re incredibly proud of. Through the extensive network that we’ve established over the years, we can now offer our full range of consulting services wherever you are based.
Unveiling Success: Impactful Case Study Highlights

Globally in-country Regulatory Support

GxP Audits – Worldwide

Regulatory Strategy IVDR / FDA
UKRP & EURP - Representative Services
Our Case Studies offer a compelling glimpse into the services Apotech provides. We showcase real examples of our expertise in action, demonstrating how we partner with clients to overcome challenges, and achieve impressive results.

Numbers that showcase our success
The story of Apotech started with our two founders: Fabien Pezous and Will Lewis. The rest, as they say, is history.
Since then, we have grown into a company that offers a global, bespoke approach to each of our services, while remaining strongly committed to our key guiding principles.
Meet our talented team
At Apotech, we wouldn’t be able to offer our extensive range of services without the knowledge and commitment that our team has.









